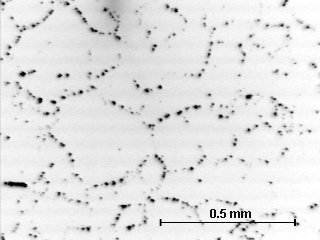
Most metals are commonly found in polycrystalline form. That is, the material is composed of many small regions, called grains, that have different orientations of the crystal structure. Between the grains are grain boundaries, which are regions of extreme disorder in the crystal structure. Due to this disorder acids and other etchants typically attack (react with) the grain boundary material first. This provides a method for making the grain boundaries visible in a optical microscope. The image below shows some typical grains in the polycrystalline copper discussed here.

The grain boundaries are visible as a series of dark spots where the acid has attacked the grain boundaries. These grains are quite large. Grains can range in size from inches (a single crystal specimen is really just a single grain) to microns (0.001 mm) in size. The strength of a polycrystalline metal increases as the grain size decreases, this is known as the Hall-Petch effect, after the scientists who first studied it.
The deformation that occurs near a notch tip in a polycrystalline material is quite different from the slip bands observed in the copper single crystal. Because each grain is oriented differently, it deforms differently than its neighbors and instead of a smooth (aside from slip bands) pattern, a very mottled and textured surface develops as shown below.
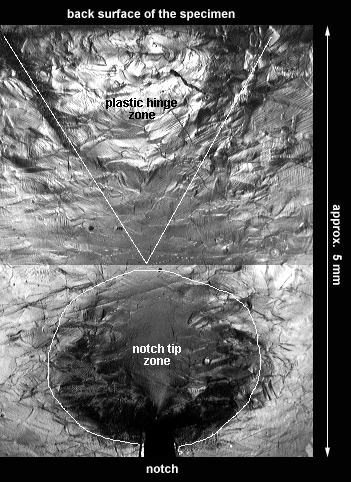
The surface is especially rough near the notch tip at the bottom of this picture where the plastic strains are the largest, this is labled as the notch tip zone. The plastic hinge at the top of the picture is due to the overall bending that occurred in the specimen. This type of feature would be present on both the top and the bottom of a plastically bent copper bar that did not have a notch.
The image above shows the surface texture when the grains are large. For materials with very small grains, the behavior at the scale of the picture above would appear to be smooth. However, the plastic strains near the notch tip would still be very different from those measured in a single crystal. This is because polycrystals behave isotropically, that is their properties are the same in all directions. Single crystals are highly anisotropic. For example the Young's modulus (the stiffness of the material in tension) of a copper single crystal varies from a low of 67 GPa (<100> direction), to a high of 192 GPa (<111> direction) depending on orientation. In contrast polycrystalline copper has the same modulus of 111 GPa in all directions.